Background:The tx landscape of R/R FL is evolving with the availability of anti-CD19 chimeric antigen receptor (CAR) T cell therapies and CD20xCD3 bispecific T-cell-engaging monoclonal antibodies (mAb). Liso-cel is an autologous, CD19-directed, 4-1BB CAR T cell therapy which recently reported a positive benefit-risk tx profile for the 3L+ tx of patients with R/R FL (Morschhauser F, et al. ICML 2023; LBA4). Mosunetuzumab, a CD20xCD3 bispecific T-cell-engaging mAb, is approved for the tx of R/R FL after 2 or more lines of therapy (LOT). In the absence of head-to-head data, we conducted an unanchored MAIC to compare the efficacy and safety of liso-cel and mosunetuzumab for the 3L+ tx of patients with R/R FL.
Methods: Unanchored MAICs were used to estimate population-adjusted relative tx effects between individual patient-level data from TRANSCEND FL (NCT04245839; data as of January 27, 2023; median follow-up of 19.3 months) and aggregate data from GO29781 (NCT02500407; data as of August 27, 2021; median follow-up of 18.3 months; N = 90). For TRANSCEND FL, the leukapheresed (intention to treat [ITT]) set (N = 114) was used for primary comparisons of efficacy endpoints: Progression-free survival (PFS), objective response rate, and complete response rate (all per independent review committee assessment). Overall survival was not analyzed due to the low rate of events. The treated efficacy set (n = 101) was used for a sensitivity analysis of efficacy comparisons. The treated set (n = 107) was used for comparisons of safety endpoints: cytokine release syndrome (CRS), neurological events (NEs), serious infections, and use of corticosteroids or tocilizumab for CRS. Baseline characteristics and outcome measures in TRANSCEND FL were redefined to align with those reported in GO29781. Data from TRANSCEND FL were weighted using a method-of-moments propensity score model to match the marginal distribution (ie, mean, variance) of clinical factors among patients from GO29781. A panel of expert clinicians selected and ranked tx effect modifiers separately for efficacy and safety endpoints. For efficacy, factors adjusted for included number of prior systemic LOTs, progression of disease ≤ 24 months from initiation of immunochemotherapy, bulky disease at screening, FL International Prognostic Index risk factor, refractory to last therapy, double refractory to an anti-CD20 antibody and alkylating agent, ECOG PS at screening, sex, and prior autologous HSCT. For safety, factors adjusted for included age, bulky disease at screening, refractory to last therapy, number of prior systemic LOTs, ECOG PS at screening, Ann Arbor stage, and sex. Hazard ratios (HR) were used to compare PFS, and odds ratios (OR) were used for response rates and safety outcomes.
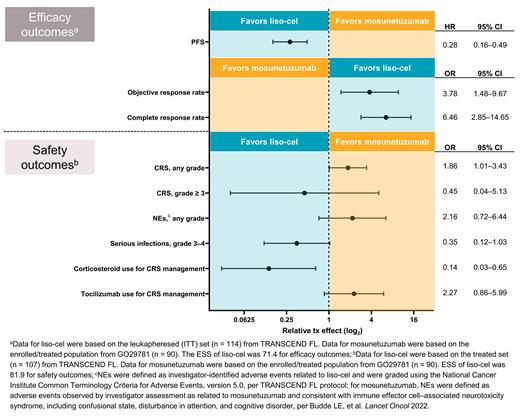
Results: After adjusting for all available factors, when compared with mosunetuzumab, liso-cel was associated with higher objective response rate (OR, 3.78 [95% CI, 1.48‒9.67]), and complete response rate (OR, 6.46 [2.85‒14.65]), and improved PFS (HR, 0.28 [0.16‒0.49]) based on an effective sample size (ESS) of 71.4 (Figure). Results were consistent across all scenario analyses, including unadjusted comparisons and sensitivity analyses using the treated efficacy set for TRANSCEND FL. Although liso-cel was associated with higher incidence of any-grade CRS (OR, 1.86 [1.01‒3.43]) and any-grade NEs (OR, 2.16 [0.72‒6.44]), it demonstrated a lower incidence of grade ≥ 3 CRS (OR, 0.45 [0.04‒5.13]) and grade 3-4 serious infections (OR, 0.35 [0.12‒1.03]) based on an ESS of 81.9 compared with mosunetuzumab. Furthermore, liso-cel was associated with reduced use of corticosteroids for CRS management (OR, 0.14 [0.03‒0.65]), while the use of tocilizumab was higher in patients treated with liso-cel (OR, 2.27 [0.86‒5.99]).
Conclusions: In this MAIC, liso-cel was associated with improved efficacy compared with mosunetuzumab. Liso-cel had a lower incidence of grade ≥ 3 CRS, grade 3-4 serious infections, and steroid use for management of CRS; however, liso-cel exhibited higher incidence of any-grade CRS, any-grade NEs, and tocilizumab use for CRS management. Safety comparisons were confounded by preventative use of corticosteroids in all patients in GO29781 and definition differences, most notably for NEs. These findings highlight a potential positive benefit-risk profile of liso-cel over mosunetuzumab as a 3L+ tx option for R/R FL.
Disclosures
Nastoupil:Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Regeneron: Honoraria; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Bonner:EVERSANA: Current Employment. Wang:EVERSANA: Current Employment. Almuallem:EVERSANA: Current Employment. Desai:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Fasan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Sanofi Genzyme: Speakers Bureau; Oncopeptides: Other: Advisory Board, Speakers Bureau. Farazi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Kumar:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Dahiya:Adaptive Biotechnologies: Consultancy; Incyte: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal